Uses
It is used in the treatment of type II diabetes. As of 2007[update], it is one of only two oral anti-diabetics in the World Health Organization Model List of Essential Medicines (the other being metformin).[1] As of 2003, in the United States, it was the most popular sulfonyurea.[2]
Additionally, recent research shows that glyburide improves outcome in animal stroke models by preventing brain swelling. A retrospective study showed that in type 2 diabetic patients already taking glyburide there was improved NIH stroke scale scores on discharge compared to diabetic patients not taking glyburide.
 | |
 | |
| Glibenclamide | |
| Systematic (IUPAC) name | |
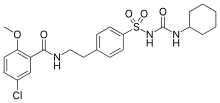
| 5-chloro-N-(4-[N-(cyclohexylcarbamoyl)sulfamoyl]phenethyl)-2-methoxybenzamide | |
| Identifiers | |
| CAS number | |
| ATC code | A10 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | C23H28ClN3O5S |
| Mol. mass | 494.004 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | Extensive |
| Metabolism | Hepatic hydroxylation (CYP2C9-mediated) |
| Half life | 10 hours |
| Excretion | Renal and biliary |
| Therapeutic considerations | |
| Licence data | |
| Pregnancy cat. | |
| Legal status | |
| Routes | Oral |








